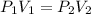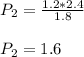Chemistry, 28.06.2019 03:30 vivianni0727p1y30v
Asample of gas has a volume of 2.4 l and a pressure of 1.2 atm. what would the pressure of the same gas sample be if the volume is reduced to 1.8 l at a constant temperature? 0.90 atm 1.6 atm 5.2 atm 3.6 atm

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3
You know the right answer?
Asample of gas has a volume of 2.4 l and a pressure of 1.2 atm. what would the pressure of the same...
Questions






Mathematics, 06.03.2020 23:34

Mathematics, 06.03.2020 23:34


Computers and Technology, 06.03.2020 23:34