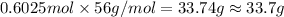And explain how to get the answer! 1. given the equation: 2na+cl2--> 2nacl if 200 grams of naci is produced, how many grams of na must be reacted with excess chlorine? a. 58.43g nab. 78.65g nac. 22.98g nad. 3.4g na2. given the equation: 2k+2h2o--> 2koh+h2 if 23.5 grams of potassium are reacted with excess water, how many grams of potassium hydroxide will be formed? a. 33.7g kohb. 56.08g kohc. 39.09g kohd. 17.99g koh

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1


Chemistry, 23.06.2019 04:00
Calculate the mass of 0.750 mol of the following substance. na3po4. , i'm not quite sure on how to set up the problem to solve! : (
Answers: 1
You know the right answer?
And explain how to get the answer! 1. given the equation: 2na+cl2--> 2nacl if 200 grams of naci...
Questions


Mathematics, 24.01.2022 22:21


Mathematics, 24.01.2022 22:21

Mathematics, 24.01.2022 22:21

Mathematics, 24.01.2022 22:21




Mathematics, 24.01.2022 22:21


Mathematics, 24.01.2022 22:21

History, 24.01.2022 22:21


English, 24.01.2022 22:21

Mathematics, 24.01.2022 22:21

Mathematics, 24.01.2022 22:21


Mathematics, 24.01.2022 22:21

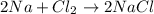
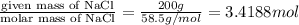
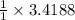 moles of Na.
moles of Na.
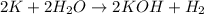
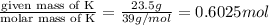
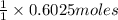 of KOH
of KOH