
Chemistry, 28.06.2019 02:30 jobucks9976
Fe3+(aq) (yellow) + scn-(aq) (colorless) fescn2+(aq) (blood-red) chloride ions are colorless. potassium ions are also colorless. the above equilibrium can be created by mixing an iron (iii) chloride solution with a potassium thiocyanate solution. based on this information and the colors in the equilibrium above answer the first two questions. 1. what color would an fecl3 solution be? 2. what color would a kscn solution be? 3. what color do you get when you mix fecl3 and kscn as shown in the video for the control test tube?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3


Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2

Chemistry, 23.06.2019 00:00
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3
You know the right answer?
Fe3+(aq) (yellow) + scn-(aq) (colorless) fescn2+(aq) (blood-red) chloride ions are colorless. potass...
Questions

Mathematics, 31.03.2021 19:10


Mathematics, 31.03.2021 19:10


Mathematics, 31.03.2021 19:10

Mathematics, 31.03.2021 19:10

Physics, 31.03.2021 19:10


History, 31.03.2021 19:10


Mathematics, 31.03.2021 19:10

Mathematics, 31.03.2021 19:10

Mathematics, 31.03.2021 19:10


Mathematics, 31.03.2021 19:10

Mathematics, 31.03.2021 19:10


Health, 31.03.2021 19:10

Mathematics, 31.03.2021 19:10

Mathematics, 31.03.2021 19:10

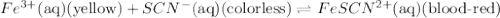
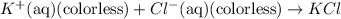
![FeCl_3+K(SCN)\rightleftharpoons KCl+[Fe(SCN)]Cl_2(blood-red)](/tpl/images/0025/5430/e3b23.png)
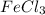 we will get blood red color solution of
we will get blood red color solution of ![[FeSCN]Cl_2](/tpl/images/0025/5430/ecffe.png) .
.

