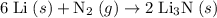Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1

Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2
You know the right answer?
Lithium and nitrogen react in a combination reaction to produce lithium nitride: 6li (s) + n2 (g) →...
Questions




Spanish, 15.04.2020 01:15

Chemistry, 15.04.2020 01:15

English, 15.04.2020 01:15



Mathematics, 15.04.2020 01:15