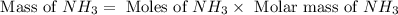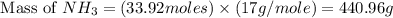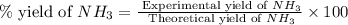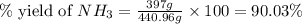Chemistry, 27.06.2019 20:30 elijahlylejamez45
25 i got the #1, just not #2 and #3. an industrial chemical company has opened a new plant that will produce ammonia (nh3). hydrogen and nitrogen gases are reacted to produce the ammonia. for the first batch of ammonia production, 475 g of nitrogen is reacted with excess hydrogen, and 397 g of ammonia are produced. • write the balanced equation for the formation of ammonia from hydrogen and nitrogen.  2nh3 • calculate the theoretical yield of ammonia. work must be shown to earn credit. • calculate the percent yield for the ammonia production. work must be shown to earn credit.
2nh3 • calculate the theoretical yield of ammonia. work must be shown to earn credit. • calculate the percent yield for the ammonia production. work must be shown to earn credit.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1
You know the right answer?
25 i got the #1, just not #2 and #3. an industrial chemical company has opened a new plant that wil...
Questions



Mathematics, 26.11.2019 01:31

English, 26.11.2019 01:31

Mathematics, 26.11.2019 01:31

English, 26.11.2019 01:31

Mathematics, 26.11.2019 01:31

Mathematics, 26.11.2019 01:31




Physics, 26.11.2019 01:31



History, 26.11.2019 01:31

Mathematics, 26.11.2019 01:31


Mathematics, 26.11.2019 01:31

Mathematics, 26.11.2019 01:31

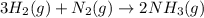
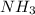 gas = 440.96 g
gas = 440.96 g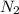 = 475 g
= 475 g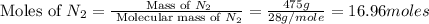
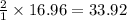 moles of
moles of