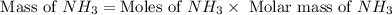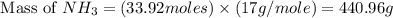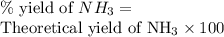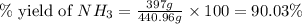Chemistry, 27.06.2019 20:30 paigesyring
25 i got the #1, just not #2 and #3. an industrial chemical company has opened a new plant that will produce ammonia (nh3). hydrogen and nitrogen gases are reacted to produce the ammonia. for the first batch of ammonia production, 475 g of nitrogen is reacted with excess hydrogen, and 397 g of ammonia are produced. • write the balanced equation for the formation of ammonia from hydrogen and nitrogen. 3h2+n2--> 2nh3 • calculate the theoretical yield of ammonia. work must be shown to earn credit. • calculate the percent yield for the ammonia production. work must be shown to earn credit.


Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which type of reaction always has an element and a compound as reactants
Answers: 1


Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
You know the right answer?
25 i got the #1, just not #2 and #3. an industrial chemical company has opened a new plant that wil...
Questions

Medicine, 14.10.2020 02:01

Mathematics, 14.10.2020 02:01


History, 14.10.2020 02:01



English, 14.10.2020 02:01


Mathematics, 14.10.2020 02:01


Mathematics, 14.10.2020 02:01



History, 14.10.2020 02:01



Mathematics, 14.10.2020 02:01


Physics, 14.10.2020 02:01

English, 14.10.2020 02:01

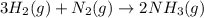
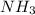 = 440.96 g
= 440.96 g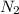 = 475 g
= 475 g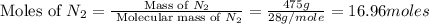
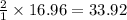 moles of
moles of