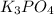Chemistry, 31.01.2020 17:47 genyjoannerubiera
Sodium metal reacts with water to produce hydrogen gas.
what best describes this reaction?
a•a single replacement reaction takes place because sodium is less reactive than hydroxide ions.
b•a double replacement reaction takes place because sodium is less reactive than hydroxide ions.
c•a double replacement reaction takes place because sodium is more reactive than hydrogen.
d•a single replacement reaction takes place because sodium is more reactive than hydrogen.
question 2(multiple choice worth 4 points)
(04.03 mc)
the table shows the nature of reactants and products formed in a certain type of chemical reaction.
nature of reactants and products
reactants products
metal + ionic compound metal + ionic compound
which of the following is true about the type of chemical reaction?
a•it is a single replacement reaction, and the anions in the two ionic compounds are different.
b•it is a single replacement reaction, and the cations in the two ionic compounds are different.
c•it is a double replacement reaction, and the anions in the two ionic compounds are different.
d•it is a double replacement reaction, and the cations in the two ionic compounds are different.
question 3(multiple choice worth 4 points)
(04.03 lc)
which of the following is a single replacement reaction?
a• ba(oh)2 + h2so4 → baso4 + 2h2o
b• 2mg + o2 → 2mgo
c• h2o+ co2 → h2co3
d• zn + h2so4 → znso4 + h2
question 4 (true/false worth 2 points)
(04.03 lc)
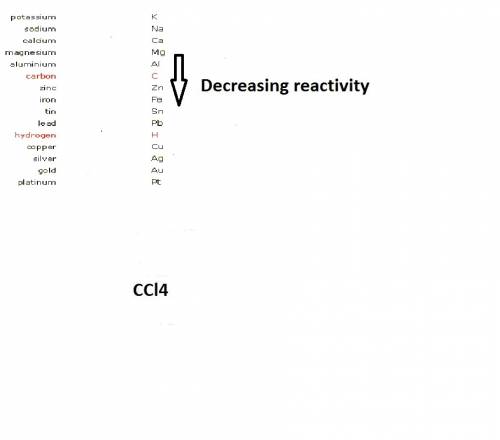
when cacl2 and zn react together, zinc (zn) can replace chlorine (cl) in the compound because zinc is higher on the periodic table.
true
false
question 5 (true/false worth 2 points)
(04.03 lc)
a double replacement reaction is a reaction in which one element replaces a similar element within a compound.
true
false
question 6(multiple choice worth 4 points)
(04.03 mc)
which of the following equations has the correct products and is balanced correctly for a reaction between na3po4 and koh?
a• na3po4 + 3koh → 3naoh + k3po4, because k retains the same charge throughout the reaction
b• na3po4 + koh → na3oh + kpo4, because k increases in charge from 1+ to 3+ when it is replaced
c• na3po4 + koh → 3naoh + k3po4, because k retains the same charge throughout the reaction
d• na3po4 + koh → na3oh + k3po4, because k increases in charge from 1+ to 3+ when it is replaced

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Since the gas in your graduated cylinder is a mixture of butane and water vapor, you must determine the partial pressure of the butane, pbutane, alone. to do this, consult a reference and record the partial pressure of the water vapor, pwater, at the temperature you recorded. use the following formula to compute the partial pressure of the butane. pbutane = atmosphere - pwater use the following combined gas law formula and compute the volume that the butane sample will occupy at stp. (hint: convert both temperatures to kelvin.) pbutane x voriginal = pstandard x vfinal troom tstandard use the following ratio and proportion formula to determine the mass of butane needed to occupy a volume of 22.4 l at stp. grams of butane you used “x” grams of butane ml of butane corrected to stp = 22,400 ml compute the theoretical molar mass of butane based on its formula and the atomic masses on the periodic table. compare your experimental results from #3 to the theoretical value of #4, computing a percent error of your findings using this formula: % error = measured value - accepted value x 100 accepted value use the following ratio and proportion formula to determine the mass of butane needed to occupy a volume of 22.4 l at stp. need asap
Answers: 1

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 21:00
Which of the following is a physical property flammability heat of combustion solubility and toxicity
Answers: 1

Chemistry, 23.06.2019 01:00
The time that is taken by neptune once around the sun is called
Answers: 1
You know the right answer?
Sodium metal reacts with water to produce hydrogen gas.
what best describes this reactio...
what best describes this reactio...
Questions





History, 19.12.2019 07:31



Mathematics, 19.12.2019 07:31




Mathematics, 19.12.2019 07:31

Mathematics, 19.12.2019 07:31


Mathematics, 19.12.2019 07:31


Biology, 19.12.2019 07:31

Mathematics, 19.12.2019 07:31


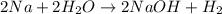 Sodium metal reacts with water to givesodium hydroxide and hydrogen gas. A single replacement reaction takes place because sodium is more reactive than hydrogen.
Sodium metal reacts with water to givesodium hydroxide and hydrogen gas. A single replacement reaction takes place because sodium is more reactive than hydrogen. 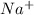 and
and 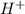 get reduced to give
get reduced to give 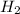 .
.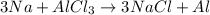 where Sodium is a metal and
where Sodium is a metal and 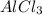 is an ionic compound. Na being more reactive than Al, displaces it from its salt solution.
is an ionic compound. Na being more reactive than Al, displaces it from its salt solution.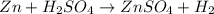
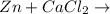 no reaction
no reaction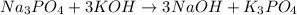 , because K retains the same charge throughout the reaction
, because K retains the same charge throughout the reaction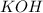 as well as
as well as