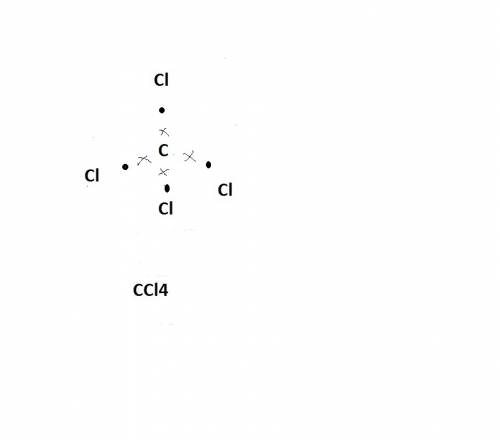
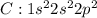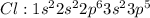Carbon tetrachloride has been widely used in the cleaning industry, in fire extinguishers, and as a refrigerant. construct an explanation of how carbon and chlorine combine to form carbon tetrachloride. a) nonmetal carbon shares valence electrons with each nonmetal chlorine forming four covalent bonds. b) nonmetal carbon loses a valence electron and chlorine metal gains a valence electron to form an ionic bond. c) carbon and chlorine are nonmetals and they shares their valence electrons to become ions and form ionic bonds. d) chlorine metal loses a valence electron to become a cation and nonmetal carbon gains a valence electron to become an anion forming a covalent bond.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
You know the right answer?
Carbon tetrachloride has been widely used in the cleaning industry, in fire extinguishers, and as a...
Questions


Mathematics, 18.07.2019 17:20

English, 18.07.2019 17:20



Mathematics, 18.07.2019 17:20




Social Studies, 18.07.2019 17:20

Chemistry, 18.07.2019 17:20



Computers and Technology, 18.07.2019 17:20






Computers and Technology, 18.07.2019 17:20