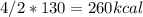Chemistry, 27.06.2019 15:30 hahalol123goaway
Calculate the enthalpy change when 4.00 mol cl2o7 is produced according to the following balanced equation: 2cl2(g) + 7o2(g) + 130kcal -> 2cl2o7(g) a. 1040 kcal b. -260 kcal c. 260 kcal d. -1040 kcal ** if you could explain it as well, that would be much appreciated if not, thats okay too its multiple choice

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

You know the right answer?
Calculate the enthalpy change when 4.00 mol cl2o7 is produced according to the following balanced eq...
Questions

History, 24.04.2020 02:03



Mathematics, 24.04.2020 02:03

Mathematics, 24.04.2020 02:03


Advanced Placement (AP), 24.04.2020 02:03




History, 24.04.2020 02:03

Mathematics, 24.04.2020 02:03


Mathematics, 24.04.2020 02:03


History, 24.04.2020 02:03



Health, 24.04.2020 02:03

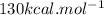
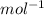 is mentioned because it is for per mole of reaction. So for 4. moles of the product
is mentioned because it is for per mole of reaction. So for 4. moles of the product 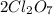 we need 4/2 moles of reaction to be used to calculate associated enthalphy change for the reaction.
we need 4/2 moles of reaction to be used to calculate associated enthalphy change for the reaction.