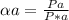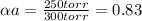Chemistry, 27.06.2019 14:30 JewelzSkullz
Substances a and b are both volatile liquids with p*a = 300 torr, p*b = 250 torr, and kb = 200 torr (concentration expressed in mole fraction). when xa = 0.9, bb = 2.22 mol kg−1, pa = 250 torr, and pb = 25 torr. calculate the activities and activity coefficients of a and b. use the mole fraction, raoult's law basis system for a and the henry's law basis system (both mole fractions and molalities) for b.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2


Chemistry, 22.06.2019 22:30
Consider a culture medium on which only gram-positive organisms such as staphylococcus aureus colonies can grow due to an elevated nacl level. a yellow halo surrounds the growth, indicating the bacterium fermented a sugar in the medium, decreasing the ph as a result and changing the color of a ph indicator chemical. this type of medium would be referred to as a differential and enrichment culture.
Answers: 2
You know the right answer?
Substances a and b are both volatile liquids with p*a = 300 torr, p*b = 250 torr, and kb = 200 torr...
Questions



History, 01.09.2019 20:10




Mathematics, 01.09.2019 20:10

Mathematics, 01.09.2019 20:10



Mathematics, 01.09.2019 20:10

Mathematics, 01.09.2019 20:10


Biology, 01.09.2019 20:10



Social Studies, 01.09.2019 20:10


Health, 01.09.2019 20:10