
Chemistry, 27.06.2019 04:30 saskiat1155
Which statement is correct for pure water? a) pure water contains equal amounts of hydroxide, [oh-], and hydronium, [h3o+], ions. b) pure water contains larger amounts of hydroxide, [oh-], ions than hydronium, [h3o+], ions. c) pure water contains larger amounts of hydronium, [h3o+], ions than hydroxide, [oh-], ions. d) pure water is an electrolyte.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 07:00
The following transition occurs at a molecular level for a substance. what transition corresponds to this change in microscopic structure? the carbon dioxide molecules on the left are in a regular, tightly packed pattern. after heating, it becomes much lower density. a. melting b. boiling c. sublimation d. freezing
Answers: 1
You know the right answer?
Which statement is correct for pure water? a) pure water contains equal amounts of hydroxide, [oh-]...
Questions




















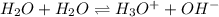
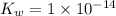
![[H_3O^+]=[OH^-]](/tpl/images/0022/0619/f28a0.png)
![K_w=1\times 10^{-14}=[H_3O^+]\times [OH^-]](/tpl/images/0022/0619/a50f9.png)
![[H_3O^+]=10^{-7}](/tpl/images/0022/0619/e211a.png)
![pH=-\log[H_3O^+]=-\log[10^{-7}] = 7](/tpl/images/0022/0619/ddc2d.png)


