
Chemistry, 27.06.2019 04:00 0IggyMarie0
Two solutions are mixed, bringing together ions of na+, po43−, v3+, and cl−. use the solubility rules to identify the composition of the salt that precipitates out of the solution. a. vpo4 b. na3po4 c. vcl3 d. nacl

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1
You know the right answer?
Two solutions are mixed, bringing together ions of na+, po43−, v3+, and cl−. use the solubility rule...
Questions

Biology, 23.02.2021 20:40

Social Studies, 23.02.2021 20:40

Mathematics, 23.02.2021 20:40

Mathematics, 23.02.2021 20:40




Mathematics, 23.02.2021 20:40

Business, 23.02.2021 20:40

Biology, 23.02.2021 20:40

Mathematics, 23.02.2021 20:40


Health, 23.02.2021 20:40

Mathematics, 23.02.2021 20:40

Mathematics, 23.02.2021 20:40



English, 23.02.2021 20:40


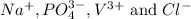
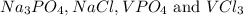
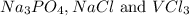
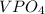 remains insoluble in water and thus, the correct answer is Option A.
remains insoluble in water and thus, the correct answer is Option A.

