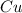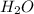Chemistry, 27.06.2019 02:30 avanelson01
An individual with an excellent sense of smell is handed a bottle of perfume. after smelling the perfume, he reports that the perfume contains a predominant smell of roses with a faint touch of vanilla. if the perfume is made from water, rose essence, and a splash of vanilla, it is most likely a. a mixture. b. a gas. c. an element. d. a compound.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1
You know the right answer?
An individual with an excellent sense of smell is handed a bottle of perfume. after smelling the per...
Questions


History, 16.01.2020 09:31

Mathematics, 16.01.2020 09:31


History, 16.01.2020 09:31





English, 16.01.2020 09:31

History, 16.01.2020 09:31

Mathematics, 16.01.2020 09:31




Physics, 16.01.2020 09:31

Mathematics, 16.01.2020 09:31


History, 16.01.2020 09:31