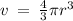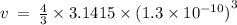Chemistry, 27.06.2019 02:00 msbanks317
Given the atomic radius of xenon, 1.3 ? , and knowing that a sphere has a volume of 4? r3/3, calculate the fraction of space that xe atoms occupy in a sample of xenon at stp.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 23.06.2019 01:00
Animals that reproduce sexually either do it through external or internal fertilization. read the following statement and decide if it is true or false. birds reproduce through external reproduction which is because the female will then be able to protect the egg.
Answers: 1

Chemistry, 23.06.2019 01:50
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1
You know the right answer?
Given the atomic radius of xenon, 1.3 ? , and knowing that a sphere has a volume of 4? r3/3, calcula...
Questions

Mathematics, 17.10.2020 08:01

Mathematics, 17.10.2020 08:01


Mathematics, 17.10.2020 08:01




Physics, 17.10.2020 08:01

Biology, 17.10.2020 08:01

English, 17.10.2020 08:01


Mathematics, 17.10.2020 08:01

Health, 17.10.2020 08:01




Mathematics, 17.10.2020 08:01

Mathematics, 17.10.2020 08:01

Biology, 17.10.2020 08:01