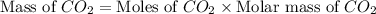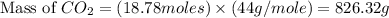Iron metal is obtained from the reaction of hematite [iron (iii) oxide, fe2o3] with carbon monoxide in a blast furnace. fe2o3 (s) + 3 co (g) > 2 fe (s) + 3 co2 (g) (a) calculate the number of grams of iron metal that can be obtained from 1.00 kg of hematite (assuming that you have enough co available for any reaction). feb) calculate the amount of co2 in grams that you you will get in this reaction, using the amount of hematite in (a). g co2

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2


Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1
You know the right answer?
Iron metal is obtained from the reaction of hematite [iron (iii) oxide, fe2o3] with carbon monoxide...
Questions

Mathematics, 18.01.2020 11:31

Chemistry, 18.01.2020 11:31


Social Studies, 18.01.2020 11:31


Mathematics, 18.01.2020 11:31

World Languages, 18.01.2020 11:31

Mathematics, 18.01.2020 11:31

Mathematics, 18.01.2020 11:31

Computers and Technology, 18.01.2020 11:31

Mathematics, 18.01.2020 11:31



Mathematics, 18.01.2020 11:31


Social Studies, 18.01.2020 11:31



Computers and Technology, 18.01.2020 11:31

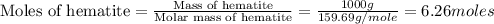
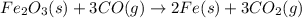
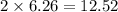 moles of iron
moles of iron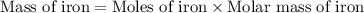
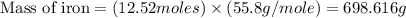
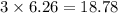 moles of carbon dioxide
moles of carbon dioxide