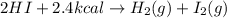Chemistry, 27.06.2019 01:30 orangeicecream
Determine the value for the following reaction. 2hi(g) + 2.4 kcal → h2(g) + l2(g) î”h = 2.4 kcal -2.4 kcal 0.0024 kcal -0.0024 kcal

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Bohr's model could only explain the spectra of which type of atoms? single atoms with one electron single atoms with more than one electron bonded atoms with one electron bonded atoms with more than one electron
Answers: 2

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3
You know the right answer?
Determine the value for the following reaction. 2hi(g) + 2.4 kcal → h2(g) + l2(g) î”h = 2.4 kcal...
Questions



Mathematics, 01.08.2019 09:00



History, 01.08.2019 09:00

History, 01.08.2019 09:00


Mathematics, 01.08.2019 09:10




Mathematics, 01.08.2019 09:10


Law, 01.08.2019 09:10

Mathematics, 01.08.2019 09:10

Mathematics, 01.08.2019 09:10



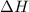 for the reaction comes out to be positive.
for the reaction comes out to be positive.