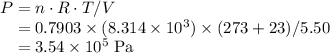Chemistry, 27.06.2019 00:30 abenjamin489
10.0 g of gaseous ammonia and 6.50 g of oxygen gas are introduced into a previously evacuated 5.50 l vessel. if the ammonia and oxygen then react to yield no gas and water vapor, what is the final gas pressure inside the vessel at 23? c

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2


Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2
You know the right answer?
10.0 g of gaseous ammonia and 6.50 g of oxygen gas are introduced into a previously evacuated 5.50 l...
Questions


Mathematics, 22.02.2021 20:20



History, 22.02.2021 20:20

Computers and Technology, 22.02.2021 20:20


Mathematics, 22.02.2021 20:20


Social Studies, 22.02.2021 20:20


Chemistry, 22.02.2021 20:20


History, 22.02.2021 20:30

Physics, 22.02.2021 20:30

Chemistry, 22.02.2021 20:30



Physics, 22.02.2021 20:30

Mathematics, 22.02.2021 20:30