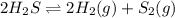Chemistry, 27.06.2019 00:30 cobyontiveros
How does the equilibrium change with the removal of hydrogen (h2) gas from this equation? 2h2s ⇌ 2h2(g) + s2(g) a. equilibrium shifts right to produce more product. b. equilibrium shifts left to produce more reactant. c. equilibrium shifts right to produce less product. d. equilibrium shifts left to produce less reactant.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1


Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 23.06.2019 06:30
Which of these natural resources is non-renewable a.corn b.wind c.geothermal d.natural gas
Answers: 2
You know the right answer?
How does the equilibrium change with the removal of hydrogen (h2) gas from this equation? 2h2s ⇌ 2h...
Questions

Mathematics, 18.03.2021 23:50



Social Studies, 18.03.2021 23:50

Health, 18.03.2021 23:50

Mathematics, 18.03.2021 23:50


Mathematics, 18.03.2021 23:50




Chemistry, 18.03.2021 23:50

Chemistry, 18.03.2021 23:50





Computers and Technology, 18.03.2021 23:50

Mathematics, 18.03.2021 23:50