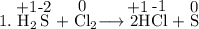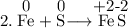Chemistry, 26.06.2019 20:30 wlackey2020
Read the following chemical equations. reaction 1: h2s + cl2 → 2hcl + s reaction 2: fe + s → fes which of the following statements is true for both the chemical equations? a. chlorine is reduced in reaction 1 and iron is reduced in reaction 2. b. chlorine is oxidized in reaction 1 and iron is oxidized in reaction 2. c. chlorine is oxidized in reaction 1 and iron is reduced in reaction 2. d. chlorine is reduced in reaction 1 and iron is oxidized in reaction 2.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
You know the right answer?
Read the following chemical equations. reaction 1: h2s + cl2 → 2hcl + s reaction 2: fe + s → fes...
Questions


Mathematics, 15.01.2021 14:00

Mathematics, 15.01.2021 14:00

Spanish, 15.01.2021 14:00

English, 15.01.2021 14:00



Mathematics, 15.01.2021 14:00



Mathematics, 15.01.2021 14:00

Mathematics, 15.01.2021 14:00

Mathematics, 15.01.2021 14:00

Mathematics, 15.01.2021 14:00

Computers and Technology, 15.01.2021 14:00



Mathematics, 15.01.2021 14:00