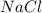Chemistry, 26.06.2019 20:30 irenecupcake4348
Which statement is correct about the reaction that occurs between calcium chloride and sodium carbonate? it is a single replacement reaction because two new compounds of calcium and sodium are formed. it is a double replacement reaction because calcium chloride and sodium carbonate exchange their non-metallic parts. it is a single replacement reaction because calcium replaces sodium within a compound as sodium is higher than calcium in the activity series. it is a double replacement reaction because the ions of calcium and sodium exchange places in an aqueous solution as calcium is higher than sodium in the activity series.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which of these sequences lists the correct order for the creation of sedimentary rock from sediment? a. deposition, burial, compaction, cementation b. burial, deposition, compaction, cementation c. compaction, deposition, burial, cementation d. cementation, deposition, burial, compaction
Answers: 1


Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1

Chemistry, 23.06.2019 11:00
The standard emf for the cell using the overall cell reaction below is +2.20 v: 2al(s) + 3i2(s) → 2ai3+(aq) + 6i-(aq) the emf generated by the cell when [ai3+] = 3.5 × 10-3 m and [i-] = 0.015 m is v. the standard emf for the cell using the overall cell reaction below is +2.20 v: 2al(s) + 3i2(s) 2ai3+(aq) + 6i-(aq) the emf generated by the cell when [ai3+] = 3.5 × 10-3 m and [i-] = 0.015 m is v. 2.36 2.24 2.21 2.51 2.04
Answers: 2
You know the right answer?
Which statement is correct about the reaction that occurs between calcium chloride and sodium carbon...
Questions

English, 29.11.2020 07:50


Chemistry, 29.11.2020 07:50


English, 29.11.2020 07:50

Advanced Placement (AP), 29.11.2020 07:50

Mathematics, 29.11.2020 07:50



Mathematics, 29.11.2020 07:50

Mathematics, 29.11.2020 07:50

Biology, 29.11.2020 07:50



Mathematics, 29.11.2020 07:50

Mathematics, 29.11.2020 07:50




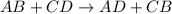
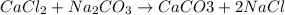
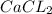 are reactants
are reactants 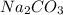 and form products by exchanging their nonmetallic parts. The products obtained in the reaction are
and form products by exchanging their nonmetallic parts. The products obtained in the reaction are 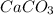 and
and