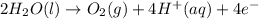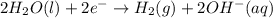Chemistry, 26.06.2019 19:30 michael2737
How does the electrolysis of water produce hydrogen gas? hydrogen cations give electrons to the anode through reduction reactions. hydrogen cations give electrons to the anode through oxidation reactions. electrons from the cathode combine with hydrogen cations through reduction reactions. electrons from the cathode combine with hydrogen cations through oxidation reactions.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Motivation cannot be developed with practice; a person either possesses it or they do not.
Answers: 1

Chemistry, 22.06.2019 00:00
Alarge marble is dropped in a graduated cylinder with 35ml of water in it.the water level increases to 49ml.what is the volume of the marble
Answers: 1

Chemistry, 22.06.2019 06:10
56.16 gregor mendel was the first scientist to use statistics to analyze scientific data. before mendel's experiments, scientists believed that organisms acquired traits from their environment and passed them on to their offspring. after mendel's discoveries were accepted, scientists realized that traits passed to offspring were the result of genes being passed from parents to offspring. this is an example of pls
Answers: 1

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2
You know the right answer?
How does the electrolysis of water produce hydrogen gas? hydrogen cations give electrons to the ano...
Questions


Mathematics, 22.11.2020 09:40

Mathematics, 22.11.2020 09:40

Mathematics, 22.11.2020 09:40


Physics, 22.11.2020 09:40


Mathematics, 22.11.2020 09:40


Mathematics, 22.11.2020 09:40

Chemistry, 22.11.2020 09:40

History, 22.11.2020 09:40


Mathematics, 22.11.2020 09:40



Physics, 22.11.2020 09:50

Mathematics, 22.11.2020 09:50


History, 22.11.2020 09:50