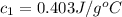Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3


Chemistry, 23.06.2019 05:00
Asolution is made by dissolving 2.3 moles of sodium chloride (nacl) in 0.155 kilograms of water. if the molal boiling point constant for water (kb) is 0.51 °c/m, what would be the boiling point of this solution? show all the steps taken to solve this problem.
Answers: 1

Chemistry, 23.06.2019 11:00
The image below shows a weather service map.. i’m not sure if is correct
Answers: 2
You know the right answer?
Apiece of metal weighing 59.047 g was heated to 100.0 °c and then put it into 100.0 ml of water (ini...
Questions




English, 25.02.2021 14:00

Mathematics, 25.02.2021 14:00


Geography, 25.02.2021 14:00


Mathematics, 25.02.2021 14:00

Mathematics, 25.02.2021 14:00

History, 25.02.2021 14:00

Mathematics, 25.02.2021 14:00


Physics, 25.02.2021 14:00

Mathematics, 25.02.2021 14:00





Biology, 25.02.2021 14:00

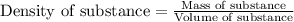
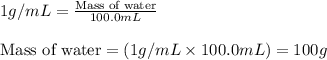
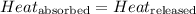
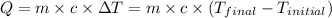
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0020/5842/09236.png) ......(1)
......(1)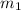 = mass of metal = 59.047 g
= mass of metal = 59.047 g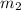 = mass of water = 100 g
= mass of water = 100 g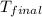 = final temperature = 27.8°C
= final temperature = 27.8°C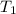 = initial temperature of lead = 100°C
= initial temperature of lead = 100°C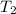 = initial temperature of water = 23.7°C
= initial temperature of water = 23.7°C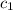 = specific heat of lead = ?
= specific heat of lead = ?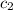 = specific heat of water= 4.186 J/g°C
= specific heat of water= 4.186 J/g°C![59.047\times c_1\times (27.8-100)=-[100\times 4.186\times (27.8-23.7)]](/tpl/images/0020/5842/d5766.png)