
Chemistry, 26.06.2019 17:30 tynitenaire
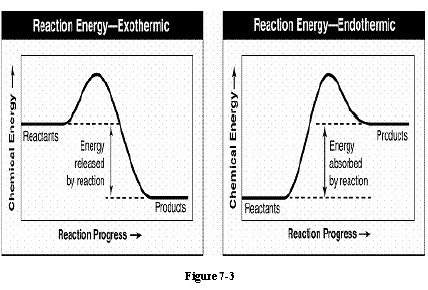
The bonds in the reactants of figure 7-3 contained 372 kj of chemical energy and the bonds in the products contained 350 kj of chemical energy. what is the amount of energy change during the reaction (show your work for full credit)? would this energy be absorbed or released? explain how you know.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 23.06.2019 03:30
In general metals get as you move from left to right across the periodic table.
Answers: 1


Chemistry, 23.06.2019 09:30
The earth's surface is (science) a: studied using seismic waves b: constantly changing over time c: only studied indirectly d: the same today as million of years
Answers: 1
You know the right answer?
The bonds in the reactants of figure 7-3 contained 372 kj of chemical energy and the bonds in the pr...
Questions

Biology, 04.07.2019 01:30

History, 04.07.2019 01:30

Mathematics, 04.07.2019 01:30


Mathematics, 04.07.2019 01:30


Computers and Technology, 04.07.2019 01:30

Mathematics, 04.07.2019 01:30

Mathematics, 04.07.2019 01:30


Mathematics, 04.07.2019 01:30

Mathematics, 04.07.2019 01:30





Mathematics, 04.07.2019 01:30

Mathematics, 04.07.2019 01:30

Mathematics, 04.07.2019 01:30

History, 04.07.2019 01:30

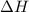 : This value is negative for exothermic reactions and positive for endothermic reactions.
: This value is negative for exothermic reactions and positive for endothermic reactions.

