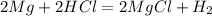Chemistry, 26.06.2019 17:00 catherinesquitieri
1. if you were to measure the mass of magnesium and hydrochloric acid before combining them in the test tube, how would that mass compare to the mass of reactants left in the test tube after the reaction? explain your answer and how it corresponds to the law of conservation of mass.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1
You know the right answer?
1. if you were to measure the mass of magnesium and hydrochloric acid before combining them in the t...
Questions




History, 02.12.2019 07:31


History, 02.12.2019 07:31


Geography, 02.12.2019 07:31

Physics, 02.12.2019 07:31

Mathematics, 02.12.2019 07:31

Chemistry, 02.12.2019 07:31


English, 02.12.2019 07:31

History, 02.12.2019 07:31


English, 02.12.2019 07:31



Mathematics, 02.12.2019 07:31