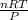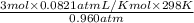1. what is the mass of 22.4 l of h2 at stp? a.) 1.01 grams b.) 2.02 grams c.) 11.2 grams d.) 22.4 grams 2. read the chemical equation. mg + hcl → mgcl2 + h2 how many liters of hydrogen gas is produced at 298 k and 0.960 atm if 3.00 moles of hydrochloric acid react with an excess of magnesium metal? a.) 38.2 liters b.) 42.6 liters c.) 66.7 liters d.) 76.4 liters

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 23.06.2019 06:30
Consider the heating curve of h2o and line segments a, b, and c. several changes are taking place at a, b, and c. all but one would be an appropriate description as e move through segments a, b and then c.
Answers: 3
You know the right answer?
1. what is the mass of 22.4 l of h2 at stp? a.) 1.01 grams b.) 2.02 grams c.) 11.2 grams d.) 22.4 g...
Questions


Mathematics, 04.03.2021 04:40



Chemistry, 04.03.2021 04:40

History, 04.03.2021 04:40

Mathematics, 04.03.2021 04:40

Arts, 04.03.2021 04:40



Chemistry, 04.03.2021 04:40

Mathematics, 04.03.2021 04:40


Mathematics, 04.03.2021 04:40



Mathematics, 04.03.2021 04:40

Mathematics, 04.03.2021 04:40

Mathematics, 04.03.2021 04:40

Social Studies, 04.03.2021 04:40

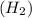 is 2.02 g/mol.
is 2.02 g/mol. 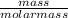
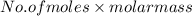
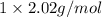
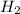 at STP is 2.02 grams.
at STP is 2.02 grams.