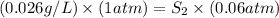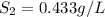1. the solubility of agno3 at 20°c is 222.0g agno3/100g h2o. what mass of agno3 can be dissolved in 250 g of water at 20°c? recall that solubility = mass of solute/ mass of solvent. 2. the solubility of methane, the major component of natural gas, in water at 20°c and 1.00 atm pressure is 0.026 g/l. if the temperature remains constant, what will be the solubility of this gas at 0.06 atm pressure? recall the relationship between solubility and pressure for gases: s1/p1 = s2/p2

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:30
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1

Chemistry, 21.06.2019 17:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. initial mass and yield sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 1

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2
You know the right answer?
1. the solubility of agno3 at 20°c is 222.0g agno3/100g h2o. what mass of agno3 can be dissolved in...
Questions

Mathematics, 30.10.2020 21:20


Geography, 30.10.2020 21:20

Engineering, 30.10.2020 21:20












Mathematics, 30.10.2020 21:20

Mathematics, 30.10.2020 21:20



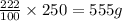
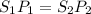 (at constant temperature)
(at constant temperature)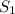 = initial solubility of methane gas = 0.026 g/L
= initial solubility of methane gas = 0.026 g/L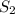 = final solubility of methane gas
= final solubility of methane gas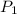 = initial pressure of methane gas = 1 atm
= initial pressure of methane gas = 1 atm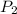 = final pressure of methane gas = 0.06 atm
= final pressure of methane gas = 0.06 atm