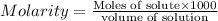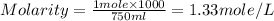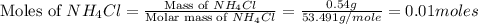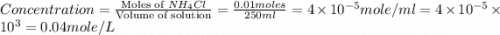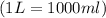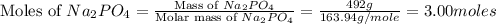Chemistry, 26.06.2019 15:30 genyjoannerubiera
1.distinguish between a 1m solution and a 1m solution. 2. calculate the molarity of 1.0 mol of kcl in 750 ml of solution. 3. what is the concentration (in m) of each of the following solutions? a. 0.54g of ammonium chloride in 250 ml of solution b. 492g of sodium phosphate in 500 ml of solution

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1

Chemistry, 23.06.2019 08:30
Of element x has 22 protons, how many electrons does it have
Answers: 1

Chemistry, 23.06.2019 15:30
Consider these four line graphs representing speed in meters/second, where each x-axis is labelled in seconds and each y-axis is labelled in meters. which line graph indicates the greatest speed at 5 seconds?
Answers: 1
You know the right answer?
1.distinguish between a 1m solution and a 1m solution. 2. calculate the molarity of 1.0 mol of kcl...
Questions

Mathematics, 08.12.2019 04:31




Social Studies, 08.12.2019 04:31



Mathematics, 08.12.2019 04:31

Mathematics, 08.12.2019 04:31

Mathematics, 08.12.2019 04:31



Chemistry, 08.12.2019 04:31







Social Studies, 08.12.2019 04:31