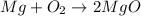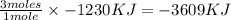Chemistry, 26.06.2019 15:00 trinity7265
Magnesium oxidizes via the reaction: 2 mg + o2 → 2 mgo the reaction has a △hrxn = -1203 kj. how much heat (in kj) is released when you completely react 3.000 moles of o2?

Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2

Chemistry, 22.06.2019 02:40
For a patient with the following pes statement and interventions, which would be the most appropriate monitoring and evaluating data? pes statement: inadequate calcium intake related to food and nutrition related knowledge deficit as evidenced by statements that the only dietary source of calcium is milk and she believes that she is lactose intolerant. patient’s nutrition prescription is for a diet providing 1200 mg calcium per day. patient was provided with in-depth nutrition education on alternative dietary and supplement sources of calcium. a. calcium intake (at subsequent visit) b. knowledge assessment by asking patient to identify food sources from menus and shopping list (at the end of the current visit) c. serum calcium (at next visit) d. both a and b e. both a and c
Answers: 2

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3
You know the right answer?
Magnesium oxidizes via the reaction: 2 mg + o2 → 2 mgo the reaction has a △hrxn = -1203 kj. how muc...
Questions

Mathematics, 19.05.2020 17:00






Mathematics, 19.05.2020 17:00

Computers and Technology, 19.05.2020 17:00



Mathematics, 19.05.2020 17:00

Mathematics, 19.05.2020 17:00

Mathematics, 19.05.2020 17:00

Mathematics, 19.05.2020 17:00



SAT, 19.05.2020 17:00


Mathematics, 19.05.2020 17:00

History, 19.05.2020 17:00

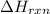 = -1230 KJ
= -1230 KJ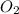 = 3 moles
= 3 moles