
1-propanol is combusted to provide heat. the reaction and the enthalpy for the reaction are shown below. c3h7oh(l)+4.5o2(g)-> 3co2(g)+4h2o(l)deltah=-2,021kj below is a list of sentences that describe a chemical reaction. choose all of the sentences that apply to the above reaction. check all that apply. view available hint(s) check all that apply. the enthalpy for 2c3h7oh(l)+9o2(g)-> 6co2(g)+8h2o(l) is 4,042 kj this process is endothermic. this process is exothermic. the enthalpy for 2c3h7oh(l)+9o2(g)-> 6co2(g)+8h2o(l) is -4,042 kj the enthalpy for 2c3h7oh(l)+9o2(g)-> 6co2(g)+8h2o(l) is 2,021 kj this chemical reaction transfers heat from the surroundings to the system. the enthalpy for 2c3h7oh(g)+9o2(g)-> 6co2(g)+8h2o(l) is -2,021 kj this chemical reaction transfers heat from the system to the surroundings.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 06:00
Oxidation-reduction reactions (often called "redox" for short) are reactions that involve the transfer of electrons from one species to another. oxidation states, or oxidation numbers, allow chemists to keep track of these electron transfers. in general, one element will lose electrons (oxidation), with the result that it will increase in oxidation number, and another element will gain electrons (reduction), thereby decreasing in oxidation number. the species that is oxidized is called the reducing agent or reductant. the species that is reduced is called the oxidizing agent or oxidant. to sum up: oxidation = increase in oxidation state = loss of electrons = reducing agent reduction = decrease in oxidation state = gain of electrons = oxidizing agent part a which element is oxidized in this reaction? fe2o3+3co→2fe+3co2 enter the elemental symbol. view available hint(s) is oxidized part b which element is reduced in this reaction? 2hcl+2kmno4+3h2c2o4→6co2+2mno2+2kcl+4h2o enter the elemental symbol. view available hint(s) is reduced
Answers: 1

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1

You know the right answer?
1-propanol is combusted to provide heat. the reaction and the enthalpy for the reaction are shown be...
Questions



Chemistry, 12.02.2021 01:00



Mathematics, 12.02.2021 01:00

English, 12.02.2021 01:00

Chemistry, 12.02.2021 01:00


Mathematics, 12.02.2021 01:00

Mathematics, 12.02.2021 01:00

Mathematics, 12.02.2021 01:00


Social Studies, 12.02.2021 01:00

Mathematics, 12.02.2021 01:00

Chemistry, 12.02.2021 01:00

Spanish, 12.02.2021 01:00

Law, 12.02.2021 01:00


Mathematics, 12.02.2021 01:00

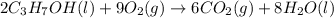 is 4,042 kJ , This process is exothermic and This chemical reaction transfers heat from the system to the surroundings.
is 4,042 kJ , This process is exothermic and This chemical reaction transfers heat from the system to the surroundings.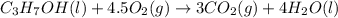
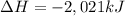
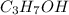 undergoes combustion to release 2,021kJ of heat.
undergoes combustion to release 2,021kJ of heat.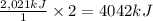 of heat.
of heat.

