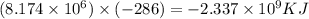Chemistry, 26.06.2019 11:00 hollymay808p0t9to
The total volume of hydrogen gas needed to fill the hindenburg was 2.00 × 108 l at 1.00 atm and 25.° c. how much energy was evolved when it burned?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

Chemistry, 23.06.2019 00:30
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1

Chemistry, 23.06.2019 01:00
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2

Chemistry, 23.06.2019 02:00
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2
You know the right answer?
The total volume of hydrogen gas needed to fill the hindenburg was 2.00 × 108 l at 1.00 atm and 25.°...
Questions





History, 11.12.2020 05:20




Mathematics, 11.12.2020 05:20

English, 11.12.2020 05:20


Mathematics, 11.12.2020 05:20

Spanish, 11.12.2020 05:20

Mathematics, 11.12.2020 05:20



Arts, 11.12.2020 05:20


Mathematics, 11.12.2020 05:20


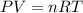
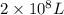
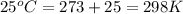
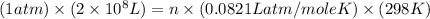
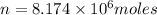
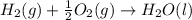
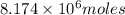 of hydrogen gas burned it evolved energy =
of hydrogen gas burned it evolved energy =