Chemistry, 26.06.2019 09:00 jasonoliva13
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.25 grams of aluminum foil in a solution of 0.40 grams of copper (ii) chloride. a single replacement reaction takes place. what are the likely observations when the reaction stops? about 0.90 grams of copper (ii) is formed, and some aluminum is left in the reaction mixture. about 0.20 grams of copper (ii) is formed, and some aluminum is left in the reaction mixture. about 0.90 grams of copper (ii) is formed, and some copper chloride is left in the reaction mixture. about 0.20 grams of copper (ii) is formed, and some copper chloride is left in the reaction mixture.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3


Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
You know the right answer?
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.25 grams...
Questions







History, 21.08.2021 02:10


Physics, 21.08.2021 02:10



Computers and Technology, 21.08.2021 02:10


English, 21.08.2021 02:10






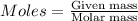
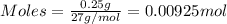
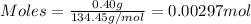
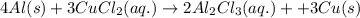
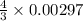 = 0.00396 moles of Aluminium.
= 0.00396 moles of Aluminium.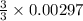 = 0.00297 moles of copper metal.
= 0.00297 moles of copper metal.