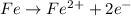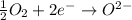Chemistry, 26.06.2019 04:00 kennedy5550
What is true when an element is oxidized? it bonds with the hydroxide ion. it loses electrons to another element. it reacts with oxygen gas. it takes electrons from another element.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

Chemistry, 23.06.2019 00:30
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3
You know the right answer?
What is true when an element is oxidized? it bonds with the hydroxide ion. it loses electrons to an...
Questions



Physics, 26.08.2020 14:01

Arts, 26.08.2020 14:01

Mathematics, 26.08.2020 14:01


English, 26.08.2020 14:01


Biology, 26.08.2020 14:01

Engineering, 26.08.2020 14:01


Social Studies, 26.08.2020 14:01

Physics, 26.08.2020 14:01

English, 26.08.2020 14:01


Mathematics, 26.08.2020 14:01

Chemistry, 26.08.2020 14:01

History, 26.08.2020 14:01

Mathematics, 26.08.2020 14:01

Mathematics, 26.08.2020 14:01