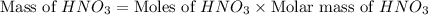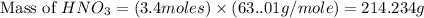Chemistry, 26.06.2019 01:30 nculberson6
Calculate the mass of 3.4 moles of nitric acid (hno3). explain the process or show your work by including all values used to determine the answer

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:50
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2

You know the right answer?
Calculate the mass of 3.4 moles of nitric acid (hno3). explain the process or show your work by incl...
Questions

Mathematics, 04.05.2020 23:30




History, 04.05.2020 23:30





Computers and Technology, 04.05.2020 23:30


Chemistry, 04.05.2020 23:30







Mathematics, 04.05.2020 23:30