
Chemistry, 25.06.2019 22:30 tatibean26
Consider the chemical equations shown here.2no2(g) > 2no(g) + o2(g)2no(g) > n2(g) + o2(g)n2(g) + 2o(g) > n2o4(g)what is the equation for the overall reaction obtained by adding these equations? 2no2(g) > n2o4(g)2n204(g) + 2no(g) > 2no2(g) + 02(g)n2 + o2(g) + 2no(g) > n2o4 (g)the answer is a

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 23.06.2019 05:30
Suppose you discovered a new element with 120 protons and 2 electrons in its outer level . i'm what group does this new element belong? what properties would you expect it to have
Answers: 1
You know the right answer?
Consider the chemical equations shown here.2no2(g) > 2no(g) + o2(g)2no(g) > n2(g) + o2(g)n2(...
Questions

Mathematics, 28.02.2020 04:01


Mathematics, 28.02.2020 04:01

Mathematics, 28.02.2020 04:01

Chemistry, 28.02.2020 04:01


Mathematics, 28.02.2020 04:01

Chemistry, 28.02.2020 04:01

Computers and Technology, 28.02.2020 04:01


Mathematics, 28.02.2020 04:01










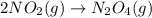
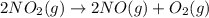 ...(1)
...(1)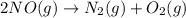 ...(2)
...(2)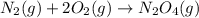 ...(3)
...(3)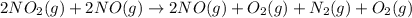
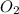 is on same side it will get added up.
is on same side it will get added up.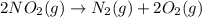 ..(4)
..(4)



