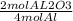Chemistry, 25.06.2019 21:30 madelyngv97
Given the following equation: 4al + 3o2 → 2al2o3. how many grams of aluminum oxide would form from reaction if 12.5 moles of aluminum burned?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 02:20
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2
You know the right answer?
Given the following equation: 4al + 3o2 → 2al2o3. how many grams of aluminum oxide would form from...
Questions

Mathematics, 01.05.2021 17:30






Biology, 01.05.2021 17:30

French, 01.05.2021 17:30







Physics, 01.05.2021 17:30

Mathematics, 01.05.2021 17:30



Mathematics, 01.05.2021 17:30

History, 01.05.2021 17:30