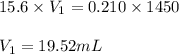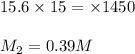Chemistry, 25.06.2019 14:00 ayowazzzgood
You have a stock solution of 15.6 m nh3. how many milliliters of this solution should you dilute to make 1450 ml of 0.210 m nh3? if you take a 15.0-ml portion of the stock solution and dilute it to a total volume of 0.600 l , what will be the concentration of the final solution?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Which orbitals form a pi bond? a.the s orbital and three p orbitals b.the s orbital and two p orbitals c.overlapping p orbitals d.overlapping hybrid orbitals
Answers: 2

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 23.06.2019 05:30
Calculate the temperature rise when 0.2g of propane is used to heat 400cm cubed of water.
Answers: 3

Chemistry, 23.06.2019 10:30
Which of the following pairs of elements is most likely to form an ionic compound? a oxygen and fluorine b sodium and aluminum c calcium and chlorine d nitrogen and sulfur
Answers: 1
You know the right answer?
You have a stock solution of 15.6 m nh3. how many milliliters of this solution should you dilute to...
Questions

Mathematics, 07.07.2019 07:00

History, 07.07.2019 07:00



Mathematics, 07.07.2019 07:00

Social Studies, 07.07.2019 07:00

Mathematics, 07.07.2019 07:00


Biology, 07.07.2019 07:00


Geography, 07.07.2019 07:00

Spanish, 07.07.2019 07:00

History, 07.07.2019 07:00



History, 07.07.2019 07:00

Biology, 07.07.2019 07:00

Mathematics, 07.07.2019 07:00


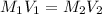
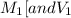 are the molarity and volume of one solution
are the molarity and volume of one solution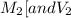 are the molarity and volume of another solution
are the molarity and volume of another solution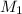 = Molarity of the stock solution = 15.6M
= Molarity of the stock solution = 15.6M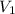 = Volume of the stock solution = ? mL
= Volume of the stock solution = ? mL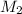 = Molarity of the diluted solution = 0.210M
= Molarity of the diluted solution = 0.210M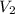 = Volume of the diluted solution = 1450 mL
= Volume of the diluted solution = 1450 mL