
Chemistry, 25.06.2019 13:30 cloeybrown
Which of the following has the greatest electronegativity difference between the bonded atoms? a. a strong acid made of hydrogen and a halogen, such as hcl b. carbon bonded to a group 6 a (16) nonmetal chalcogen, such as in co c. a diatomic gas, such as nitrogen (n2).d. a group 1 alkali metal bonded to iodide, such as nai.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 23.06.2019 01:00
Which statement is true regarding the diagram of circle p? the sum of y and z must be 2x. the sum of y and z must be x. the difference of z and y must be 2x. the difference of z and y must be x
Answers: 1

Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1

Chemistry, 23.06.2019 02:30
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2
You know the right answer?
Which of the following has the greatest electronegativity difference between the bonded atoms? a. a...
Questions

English, 14.06.2021 14:00

Mathematics, 14.06.2021 14:00

World Languages, 14.06.2021 14:00

French, 14.06.2021 14:00

Mathematics, 14.06.2021 14:00


Computers and Technology, 14.06.2021 14:00

Mathematics, 14.06.2021 14:00

Mathematics, 14.06.2021 14:00

English, 14.06.2021 14:00

English, 14.06.2021 14:00


Mathematics, 14.06.2021 14:00

World Languages, 14.06.2021 14:00

Computers and Technology, 14.06.2021 14:00


Social Studies, 14.06.2021 14:00


Mathematics, 14.06.2021 14:00

Social Studies, 14.06.2021 14:00

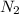 = Electronegativity value of nitrogen - electronegativity value of nitrogen
= Electronegativity value of nitrogen - electronegativity value of nitrogen

