Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
You know the right answer?
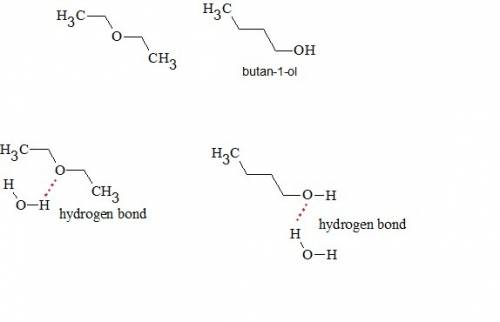
Diethyl ether and butan-1-ol are isomers, and they have similar solubilities in water. their boiling...
Questions

Mathematics, 15.01.2021 17:50

Mathematics, 15.01.2021 17:50


Mathematics, 15.01.2021 17:50

Mathematics, 15.01.2021 17:50


Chemistry, 15.01.2021 17:50

Mathematics, 15.01.2021 17:50

Mathematics, 15.01.2021 18:00




Mathematics, 15.01.2021 18:00

Mathematics, 15.01.2021 18:00

English, 15.01.2021 18:00

Mathematics, 15.01.2021 18:00