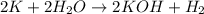When potassium metal is placed in water, a large amount of energy is released as potassium hydroxide and hydrogen gas are produced in the reaction 2k(s) + 2h2o(l) → 2koh(aq) + h2(g). your lab partner says this is a redox reaction and a combustion reaction. do you agree? defend your answer by explaining whether or not it meets the requirements of each type of reaction.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1
You know the right answer?
When potassium metal is placed in water, a large amount of energy is released as potassium hydroxide...
Questions

Geography, 04.02.2020 04:44

Social Studies, 04.02.2020 04:44

Social Studies, 04.02.2020 04:44

Mathematics, 04.02.2020 04:44



History, 04.02.2020 04:45


History, 04.02.2020 04:45

History, 04.02.2020 04:45

Geography, 04.02.2020 04:45

Mathematics, 04.02.2020 04:45


Social Studies, 04.02.2020 04:45


History, 04.02.2020 04:45

English, 04.02.2020 04:45