Chemistry, 25.06.2019 12:00 alejandra216
Amolecule contains four bonded pairs of electrons and zero lone pairs. what is the name of the molecular geometry? a. linear b. trigonal planar c. trigonal pyramidal d. tetrahedral

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 07:30
The scheme below is from a series of reactions that are part of a synthesis of vitamin a. answer the following questions with reference to this scheme. (i) what is "reagent a"? (ii) draw a step-by-step mechanism which explains the formation of compound c from compound b (iii) which reagents would you use to form compound e from compounds c and d (reagents b and c)? for each reagent suggested above in (ii) explain the role of the reagent in the reaction to (iv) form compound e. you may wish to do this by drawing a mechanism. 1. addition of reagent a но reagent a 2. н,о" thо oh нон-с compound a. compound b. compound c .ch-оh 1. reagent b "сно 2. reagent c сh oh compound e. compound d.
Answers: 2

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1
You know the right answer?
Amolecule contains four bonded pairs of electrons and zero lone pairs. what is the name of the molec...
Questions

Physics, 05.11.2020 02:10

Mathematics, 05.11.2020 02:10

English, 05.11.2020 02:10


Mathematics, 05.11.2020 02:10

Mathematics, 05.11.2020 02:10

English, 05.11.2020 02:10







Mathematics, 05.11.2020 02:10


Chemistry, 05.11.2020 02:10

Physics, 05.11.2020 02:10

Mathematics, 05.11.2020 02:10

Mathematics, 05.11.2020 02:10

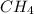 has tetrahedral geometry.
has tetrahedral geometry.