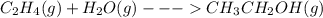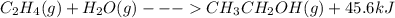When c2h4(g) reacts with h2o(g) to form ch3ch2oh(g) , 45.6 kj of energy are evolved for each mole of c2h4(g) that reacts. write a balanced thermochemical equation for the reaction with an energy term in kj as part of the equation. note that the answer box for the energy term is case sensitive. use the smallest integer coefficients possible and put the energy term (including the units) in the last box on the appropriate side of the equation. if a box is not needed, leave it blank. + + +?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:30
Jewelweed, a flowering plant, has seed pods that burst open when touched and forcefully eject their seeds. these structures are favorable because they a. can cause genetic changes to occur. b. prevent germination within the seed pod. c. aid in the dispersal of the species. d. attract insects that aid in pollination.
Answers: 3

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

You know the right answer?
When c2h4(g) reacts with h2o(g) to form ch3ch2oh(g) , 45.6 kj of energy are evolved for each mole of...
Questions

English, 03.12.2019 15:31


World Languages, 03.12.2019 15:31



Physics, 03.12.2019 15:31




English, 03.12.2019 15:31

Chemistry, 03.12.2019 15:31

Biology, 03.12.2019 15:31



Mathematics, 03.12.2019 15:31

Health, 03.12.2019 15:31

Mathematics, 03.12.2019 15:31



Mathematics, 03.12.2019 15:31