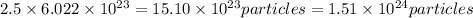Chemistry, 25.06.2019 05:30 svandewalle
How many o2 particles are in 2.50 of o2 at standard temperature and pressure (stp)? a. 4.15 x 10^22 particles b. 2.41 x 10^23 particles c. 5.02 x 10^23 particles d. 1.51 x 10^24 particles can you explain the answer ?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2
You know the right answer?
How many o2 particles are in 2.50 of o2 at standard temperature and pressure (stp)? a. 4.15 x 10^22...
Questions

History, 13.01.2021 07:40


English, 13.01.2021 07:40


French, 13.01.2021 07:40


Social Studies, 13.01.2021 07:40



English, 13.01.2021 07:40

Social Studies, 13.01.2021 07:40

World Languages, 13.01.2021 07:40





Mathematics, 13.01.2021 07:40


Mathematics, 13.01.2021 07:40

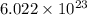 number of atoms.
number of atoms.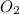 gas:
gas: