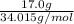Chemistry, 25.06.2019 05:00 eyeneedalife
How many moles are in 17.0 grams of h2o2? 0.284 mol h2o2 0.385 mol h2o2 0.500 mol h2o2 0.730 mol h2o2

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:50
Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation: 3no2(g)+h2o(l)→2hno3(l)+no(g) part a suppose that 4.2 mol no2 and 0.50 mol h2o combine and react completely. which reactant is in excess? express your answer as a chemical formula. nothing
Answers: 1

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1
You know the right answer?
How many moles are in 17.0 grams of h2o2? 0.284 mol h2o2 0.385 mol h2o2 0.500 mol h2o2 0.730 mol h2...
Questions


Physics, 02.12.2020 01:00


History, 02.12.2020 01:00




English, 02.12.2020 01:00


History, 02.12.2020 01:00

Health, 02.12.2020 01:00


Computers and Technology, 02.12.2020 01:00



Mathematics, 02.12.2020 01:00


Arts, 02.12.2020 01:00


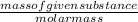
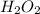 is 34.015 g/mol.
is 34.015 g/mol.