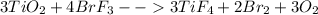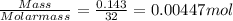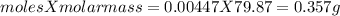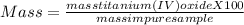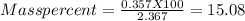Chemistry, 25.06.2019 02:00 Gearyjames8
One method for determine the purity of a sample of titanium ( iv) oxide, an important industrial chemical, is to combine the sample with bromine trifluoride to produce titanium ( iv) fluoride, liquid bromine , and oxygen has. suppose 2.367g of an impure sample( impure meaning that the sample has titanium (iv) oxide as well as other “ stuff” in it ) evolves 0.143 g of oxygen gas. what is the mass percent of titanium ( iv) oxide in the impure sample?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which feature do highland climates have that lower elevation areas do not?
Answers: 1


Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 23:00
What is the mass of naoh that would have to be added to 500 ml of a solution of 0.20 m acetic acid in order to achieve a ph of 5.0?
Answers: 1
You know the right answer?
One method for determine the purity of a sample of titanium ( iv) oxide, an important industrial che...
Questions



Social Studies, 28.05.2021 01:00

Mathematics, 28.05.2021 01:00

Computers and Technology, 28.05.2021 01:00


Mathematics, 28.05.2021 01:00


Mathematics, 28.05.2021 01:00



Mathematics, 28.05.2021 01:00

Mathematics, 28.05.2021 01:00

Mathematics, 28.05.2021 01:00

Mathematics, 28.05.2021 01:00

Mathematics, 28.05.2021 01:00



Mathematics, 28.05.2021 01:00