
In an experiment, two unknown compounds (one an alcohol and the other an ether) of equal molecular mass were dissolved in water. the result of the experiment is shown in the table. solubility comparison unknown compound solubility (g/100 ml water) unknown compound a--> solubility is 7.7 unknown compound b--> solubility is 6.8 which of the following correctly explains the identity of compound b and its solubility? a. it is an alcohol because the lack of hydrogen bonding between the oh^- make is less soluble b. it is an ether because it is unable to to form a hydrogen bond, so it is less soluble than water c. it is an ether because in the absence of a highly electronegative atom like o, f, or n, it lacks hydrogen bonding and thus makes it less soluble d. it is an alcohol because dispersion forces between the oh^- group and organic chain make it less soluble.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 01:10
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1
You know the right answer?
In an experiment, two unknown compounds (one an alcohol and the other an ether) of equal molecular m...
Questions

Computers and Technology, 22.06.2019 02:00

Advanced Placement (AP), 22.06.2019 02:00

Mathematics, 22.06.2019 02:00



Social Studies, 22.06.2019 02:00

Arts, 22.06.2019 02:00



Computers and Technology, 22.06.2019 02:00



Computers and Technology, 22.06.2019 02:00


Mathematics, 22.06.2019 02:00



Mathematics, 22.06.2019 02:00

Computers and Technology, 22.06.2019 02:00

History, 22.06.2019 02:00

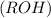 are more soluble in water as they can form hydrogen bonding with water whereas ethers
are more soluble in water as they can form hydrogen bonding with water whereas ethers 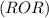 are less soluble as they do not form hydrogen bonds with water.
are less soluble as they do not form hydrogen bonds with water.

