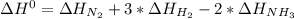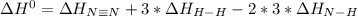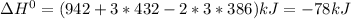Chemistry, 24.06.2019 21:30 gwoodbyrne
Given: n2 + 3h2 → 2nh3 bond bond energy (kj/mol) n≡n 942 h–h 432 n–h 386 use the bond energies to calculate the change in enthalpy for the reaction. the enthalpy change for the reaction is kilojoules.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:00
Note the ph and poh values labeled with letters on the ph scale below. based on log rules and the way ph is calculated, what is the difference in [oh– ] concentration between point a and point b. a) 10^1 b) 10^5 c) 10^6 d) 10^7
Answers: 1

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1
You know the right answer?
Given: n2 + 3h2 → 2nh3 bond bond energy (kj/mol) n≡n 942 h–h 432 n–h 386 use the bond energies to c...
Questions


Mathematics, 18.02.2020 20:18




English, 18.02.2020 20:18

Mathematics, 18.02.2020 20:19





Chemistry, 18.02.2020 20:19



Computers and Technology, 18.02.2020 20:19





Mathematics, 18.02.2020 20:19

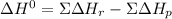 (2)
(2)