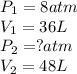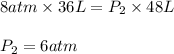Chemistry, 24.06.2019 16:00 gldven7636
When 6.0 mol of oxygen are confined in a 36l vessel at 196°c, the pressure is 8atm. what is the new pressure for oxygen expands at constant temperature to fill 48l

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:40
Which of the following pressures is equal to 760 mm hg? 2.0 atm 101.3 pa 101,300 kpa 101,300 pa
Answers: 2

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3
You know the right answer?
When 6.0 mol of oxygen are confined in a 36l vessel at 196°c, the pressure is 8atm. what is the new...
Questions


Chemistry, 02.11.2020 09:00

English, 02.11.2020 09:00

English, 02.11.2020 09:00

Chemistry, 02.11.2020 09:00



Mathematics, 02.11.2020 09:00

Mathematics, 02.11.2020 09:00

Mathematics, 02.11.2020 09:00

Mathematics, 02.11.2020 09:00


Mathematics, 02.11.2020 09:00

English, 02.11.2020 09:00

Mathematics, 02.11.2020 09:00


Mathematics, 02.11.2020 09:00



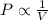
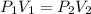
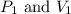 are the initial pressure and volume of the gas.
are the initial pressure and volume of the gas.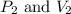 are the final pressure and volume of the gas.
are the final pressure and volume of the gas.