
Chemistry, 24.06.2019 08:30 theyycraveenaee
Match the name of each compound to its chemical formula. use the table to you. options: nitric acid sulfurous acid hypochlorous acid ammonia sulfuric acid to h2so3 nh3 hno3 h2so4 hclo

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1

Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1
You know the right answer?
Match the name of each compound to its chemical formula. use the table to you. options: nitric aci...
Questions



English, 23.02.2021 14:00

English, 23.02.2021 14:00

English, 23.02.2021 14:00

English, 23.02.2021 14:00


Computers and Technology, 23.02.2021 14:00

Mathematics, 23.02.2021 14:00

English, 23.02.2021 14:00



History, 23.02.2021 14:00

Mathematics, 23.02.2021 14:00

Mathematics, 23.02.2021 14:00

Mathematics, 23.02.2021 14:00


Chemistry, 23.02.2021 14:00


English, 23.02.2021 14:00

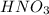
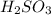
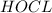
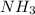
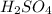
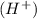 when dissolved in water or accepts a pair of electrons.
when dissolved in water or accepts a pair of electrons.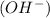 when dissolved in water or donates a pair of electrons.
when dissolved in water or donates a pair of electrons.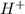 ions in water and ammonia is a base it donates its lone pair of electrons.
ions in water and ammonia is a base it donates its lone pair of electrons.

