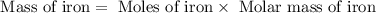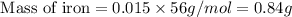Chemistry, 24.06.2019 07:30 andrewjewell2005
Achemist determined by measurements that 0.015 moles of iron participated in a chemical reaction. calculate the mass of iron that participated in the chemical reaction. round your answer to 2 significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 07:30
Which of the following statements best explains why chemistry is testable a) it can measure data by experiments b) it cannot add new evidence c) it cannot be verified d) it is biased
Answers: 1
You know the right answer?
Achemist determined by measurements that 0.015 moles of iron participated in a chemical reaction. ca...
Questions

English, 09.12.2020 14:00

Chemistry, 09.12.2020 14:00


Geography, 09.12.2020 14:00

Mathematics, 09.12.2020 14:00


Mathematics, 09.12.2020 14:00

History, 09.12.2020 14:00

Health, 09.12.2020 14:00

World Languages, 09.12.2020 14:00

History, 09.12.2020 14:00

Mathematics, 09.12.2020 14:00

Mathematics, 09.12.2020 14:00

History, 09.12.2020 14:00

Mathematics, 09.12.2020 14:00



Social Studies, 09.12.2020 14:00

Biology, 09.12.2020 14:00