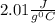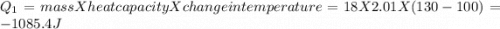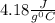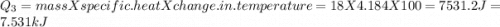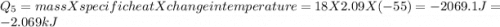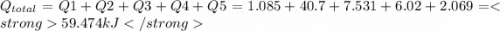Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

You know the right answer?
How much heat (in kj) is evolved in converting 1.00 mol of steam at 130.0 ∘c to ice at -55.0 ∘c? th...
Questions

Mathematics, 16.10.2020 16:01

Biology, 16.10.2020 16:01

History, 16.10.2020 16:01

History, 16.10.2020 16:01

Mathematics, 16.10.2020 16:01


Mathematics, 16.10.2020 16:01


Mathematics, 16.10.2020 16:01


Computers and Technology, 16.10.2020 16:01


Mathematics, 16.10.2020 16:01


History, 16.10.2020 16:01

Advanced Placement (AP), 16.10.2020 16:01

Mathematics, 16.10.2020 16:01


Mathematics, 16.10.2020 16:01

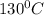 to
to 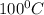 = Q1
= Q1![0^{0}C[/tex=Q3]4) conversion of water to ice=Q45) cooling of ice from [tex]0^{0}C](/tpl/images/0009/5828/c5d88.png) to
to 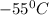 =Q5
=Q5